Abstract
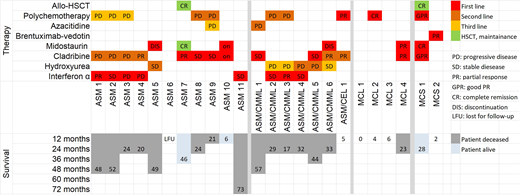
Aggressive systemic mastocytosis (ASM), mast cell leukemia (MCL) and mast cell sarcoma (MCS) are rare, life-threatening mast cell disorders, characterized by an aggressive clinical course, drug resistance and a poor survival. Established disease-modifying therapies include interferon-alpha (INF-A), cladribine (2CdA) and midostaurin (PKC412). However, these treatment approaches are unable to exert curative effects in advanced mastocytosis. Intensive therapy, including poly-chemotherapy and hematopoietic stem cell transplantation (HSCT) have also been suggested for patients with rapidly progressing ASM and MCL (Ustun et al, J Clin Oncol 2014;32:3264-3274). However, little is known about long-term outcome and survival in these patients. We analyzed the clinical course and treatment responses in 24 patients with ASM, MCL and MCS seen at the Medical University of Vienna between May 1994 and December 2017. According to World Health Organization criteria, patients were diagnosed as ASM (n=11), ASM with associated chronic myelomonocytic leukemia (ASM-CMML, n=6), ASM with associated chronic eosinophilic leukemia (ASM-CEL, n=1), MCL (n=4) and MCS (n=2). The median age at diagnosis was 59 years (range 21-90 years) and the f:m ratio was 1:2.4. Patients received first-line therapy with INF-A (3x106 units three times a week, n=8), 2-CdA (0.13 mg/kg, days 1-5, n=5); CLAG (cladribine 5mg/m2, days 1-5, ARA-C 2g/m2 day 1-5, G-CSF, 300 µg from day 6 until recovery; n=2), alternating 2CdA and midostaurin (n=2), midostaurin alone (2x100 mg/day, n=2), FLAG (fludarabine, 30mg/m², days 1-5; ARA-C 2 g/m² days 1-5; G-CSF 300µg from day 6 until recovery, n=1) and brentuximab-vedotin (1.6 mg/kg every 3 weeks, n=1). In 3 patients (ASM, n=1; MCL, n=2) no therapy could be administered because of poor performance status. Detailed information on therapies and responses in our patients are provided in Figure 1. In 17 of 21 patients (81%) a response to therapy was observed, namely a complete remission (CR) in one female MCL patient, age 54 yrs (years) receiving 2 cycles of FLAG; a good partial response (GPR) of the remaining tumor mass (after surgery) in one female patient with MCS aged 33 yrs after 2 cycles of CLAG; a partial response (PR) in 5 additional patients; and a stable disease (SD) in 9 patients (Figure 1). Two patients, one female ASM patient, age 54 yrs with PR after 6 cycles of 2CdA and one female patient with MCS, age 30 yrs, with a GPR after CLAG, underwent HSCT and achieved long-term CR under maintenance with midostaurin. Median survival until loss of response to first-line therapy was 20 months (range: 3-73 months). Interestingly, in all 4 patients who had received both 2CdA and midostaurin, no progression of the disease occurred. In the follow up, 3 patients are still alive, 7 developed a secondary acute myeloid leukemia (sAML) and in one patient with ASM-CMML, the CMML component progressed without overt AML. Three patients died due to unrelated (non-SM) events (age at death: 93, 90, and 67 yrs). The patient with CR following FLAG had a relapse of MCL after 4 months. In 3 patients receiving IFN-A, the disease showed progression under therapy and in 2 patients receiving midostaurin, therapy had to be withdrawn due to treatment-related toxicity prior to response evaluation. Interestingly, the risk to develop sAML was significantly higher in patients with ASM/CMML or ASM/CEL (71.4%) compared to patients with ASM (27.2%) or MCL/MCS without an associated hematologic neoplasm (0%) (p<0.05). Salvage therapy in patients with progressive disease (PD or toxicity under first-line therapy, n=4; PD/relapse following response to first-line therapy in patients eligible for further therapy, n=10) included 2CdA, polychemotherapy, INF-A, azacitidine and hydroxyurea. The median number of treatment lines of salvage therapy was 1.7 (range 1-4) and resulted in a median duration of response of 8 months (range: <1 to 44 months). The median survival of patients developing sAML was 4 months and was markedly shorter compared to patients without sAML (median survival 13 months). In conclusion, treatment of ASM, MCL and MCS remains a clinical challenge and unmet treatment-need. The combination of conventional therapy such as chemotherapy with novel tyrosine kinase inhibitors and/or HSCT may be a new promising approach in these patients and may lead to cure in some of them. However, controlled studies are necessary to confirm this hypothesis.
Valent:Incyte: Honoraria; Pfizer: Honoraria; Novartis: Honoraria. Sperr:Novartis: Honoraria; Pfizer: Honoraria; Daiichi Sankyo: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal